Table of Contents
Earth’s Atmosphere
The atmosphere is a mixture of many gases which support life on earth. The earth’s atmosphere provides a conducive environment for us.
Evolution of the earth’s Atmosphere
- The earth came into existence about 4.6 billion years ago.
- It composed of gases, particles of interstellar dust and ice lumped together to form a larger mass.
- Initially, the surface temperature of the earth was quite hot, having a temperature of about 8000⁰C.
- By about 4.4 billion years ago, the surface had cooled enough for the solidification of magma into a solid crust.
- This cooling also resulted in the release of gases from the Lithosphere.
- These gases mainly consist of Hydrogen and Helium.
- Due to their high temperature, the gases overcome gravity and escape to space.
Main gases of the Primitive earth’s atmosphere.
Stage-1. (4.4 billion years ago)
- At the first stage, the density of the earth’s atmosphere was 10 to 20 times higher than today’s atmosphere.
Stage-2. (3.8 billion years ago)
- At this stage, the atmospheric temperature was cool enough to allow for the condensation of water vapours.
- Rain events became common during this phase.
- Because of the high temperature in the lower atmosphere, the rain evaporated before it hit the ground.
- Due to lower temperature, the ground surface became cool enough to allow for the accumulation of water. This led to the formation of rivers, lakes and oceans.
- The oxygen came into being in the atmosphere through the chemical breakdown of the water by sunlight (Photo-dissociation).
Stage-3. (3.6 billion year ago to present)
- The emergence of living organism around 3.6 billion years ago was important in the creation of Oxygen and Ozone.
- The last period of atmosphere evolution is known as the Living Atmosphere.
Composition of earth’s Atmosphere
There are twelve most important gases found in the Earth’s atmosphere. Of all the gases, Nitrogen, O2, CO2, Methane, Nitrous-Oxide and Ozone are very important for the earth’s atmosphere.
| Name of gas | Chemical formula | Percentage by volume |
| Nitrogen | N2 | 78.08% |
| Oxygen | O2 | 20.95% |
| Argon | Ar | 0.93% |
| Carbon Dioxide | CO2 | 0.0376% |
| Neon | Ne | 0.0018% |
| Helium | He | 0.00052% |
| Methane | CH4 | 0.00017% |
| Hydrogen | H2 | 0.00006% |
| Nitrous-Oxide | N2O | 0.00003% |
| Xenon | Xe | 0.000009% |
| Ozone | O3 | 0.000004% |
Nitrogen
- It is removed from the atmosphere and deposited at the earth’s surface by nitrogen-fixing bacteria.
- Being chemically inactive, it does not easily enter into chemical union with other substance.
- Nitrogen returns to the atmosphere by the process of denitrification.
{Denitrification- a chemical process in which the solid nitrogen transforms into nitrogen gases (N2 and N2O) by the bacterial action}.
Oxygen
- Due to photosynthesis and respiration oxygen get exchange between atmosphere and biosphere.
Methane
- The sources of Methane addition include rice cultivation, domestic grazing of animals, termites, landfills, oil and gas extraction.
- The anaerobic action in the paddy fields results in the formation of methane gas.
- More than 60% of all the paddies are found in the Indian subcontinent and China.
- By the process of herbaceous digestion, grazing animals release methane in the biosphere.
- Through the ingestion of plant material, termites also release methane.
Nitrous-Oxide
- It is a greenhouse gas.
- It leads to artificial fertilization of the ecosystem.
- This fertilization may lead to forest extinction and eutrophication of aquatic habitats.
- The major sources of Nitrous-Oxide may include land-use conversion, combustion of fossils, burning of biomass and soil fertilization.
Ozone
- About 97% of the ozone is formed in the Stratosphere at an altitude of 10 to 50 km above the earth’s surface.
- It protects the earth’s surface from the burning Ultra-violet rays.
- Ozone is also found in and around the cities.
- Most of the ozone is created as a by-product of photochemical smog by humans.
Water-vapour
- It helps to warm the earth’s atmosphere.
- The condensation of vapours creates precipitation, providing needed fresh water for organisms.
- Due to the latent heat of energy exchange, water vapour redistributes the heat energy on the earth.
Dust particles
- Dust particle includes all the solid particles present in the air.
- They are held in suspensions in the lower layers of the atmosphere.
- Dust particles are important for scattering; by way of scattering, they contribute the varied colours.
- They also act as hygroscopic nuclei for condensation of water vapour; thus, they play a major role in the formation of clouds and fogs.
Structure of the earth’s atmosphere
The atmosphere having a homogeneous composition in the lower part is called Homosphere. The Heterogenous composition in the upper part is known as heterosphere.
The atmosphere can be divided into the following layers
| S. NO. | Layers of atmosphere | Vertical extent (in kilometres) |
| 1. | Troposphere | 10-14 km |
| 2. | Stratosphere | 14-30 km |
| 3. | Ozonosphere | 30-50 km |
| 4. | Mesosphere | 50-80 km |
| 5. | Thermosphere or Ionosphere | 80-400 km |
| 6. | Exosphere | 400-1000 km |
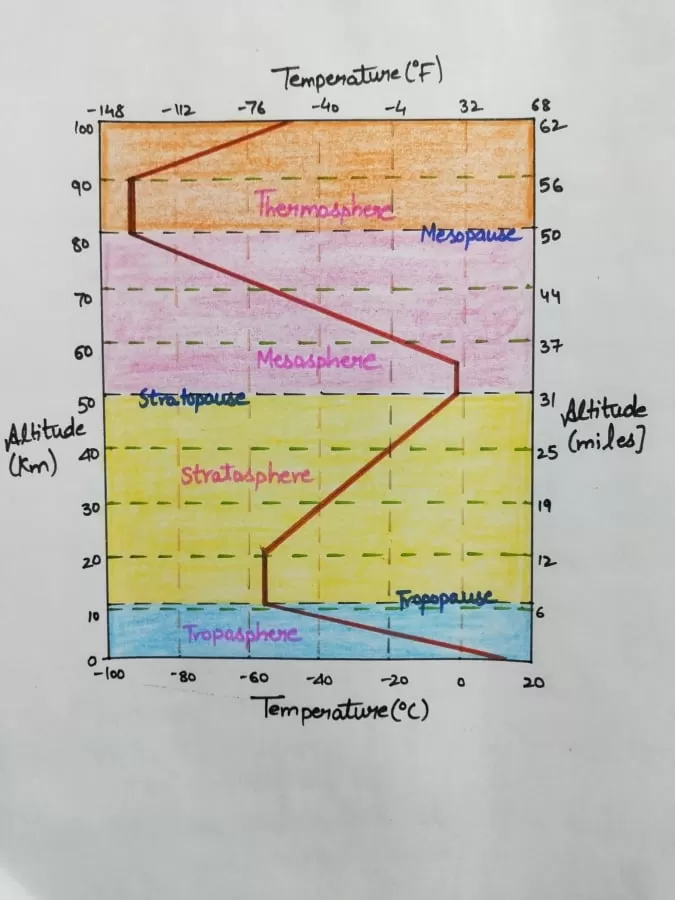
Troposphere
- It is the lowermost layer of the atmosphere in which we live.
- Most of the clouds form in this layer.
- It contains about 75% of the total gaseous, moisture and dust particles.
- The average height of this layer is about 14km above sea level, but its height over poles and equator is about 8km and 16km respectively.
- The troposphere is also called the “Convective region.
- Various clouds, thunderstorms as well as cyclones and anticyclones occur in this layer.
- Another important characteristic of the troposphere is that the wind velocities increase with height and attain the maximum at the top.
- There is a decrease of temperature with increasing elevation at a means lapse rate of about 6.5oc/km.
- At the top of the troposphere, there is a shallow layer of atmosphere separating it from Stratosphere. This shallow layer is known as the Tropopause.
- It is interesting to note that the lowest temperature in the troposphere is found directly over the equator and not above poles.
- The tropopause marks the upper limit of the transfer of atmospheric properties by large scale vertical turbulence and mixing.
- The tropopause is characterized by a sharp temperature inversion.
Stratosphere
- The lower stratosphere is isothermal that is the temperature in this part does not change with attitude or there is a slight increase in temperature.
- The lower part extends up to 20km of elevation from the earth’s surface.
- Cirrus clouds, called the mother of pearl clouds, occasionally form in the lower stratosphere.
- Beyond 20km there is gradual temperature increase up to 30km.
- The thickness of the stratosphere is highest at the poles.
- The upper boundary of the stratosphere is called the stratopause.
- Above the stratopause, there is a steep rise in temperature.
Ozonosphere (chemosphere)
- There is a maximum concentration of ozone, between 30km to 50km above the surface.
- Because of the concentration of ozone in this layer, it is called the ozonosphere. Its existence came to be known from the “studies of Meteors”.
- The formation of ozone takes place in the upper stratosphere.
- In this layer, the temperature increases with altitude at a rate of 50C/km.
- The maximum temperature in this layer is a little higher than that at the earth’s surface.
- The ozone layer acts as a filter for the ultraviolet rays of the sun.
- According to the world meteorological organization, continued release of “Chlorofluoro Carbon will result in the significant reduction in the ozone layer.
Mesosphere
- In this layer the temperature decreases with height.
- The upper boundary of this layer is called the Mesosphere.
- Here temperature reaches a minimum of about -100 degree centigrade at a height of about 80km above the earth’s surface.
Ionosphere and Thermosphere
- In this layer, the ionization of the atmosphere begins to occur.
- Scientist came to know about this ionized layer by using radio waves.
- The credit for the discovery of this layer goes to “Kennelly and Heaviside”.
- In this layer, the ionization of molecules and atoms occurs mainly as a result of UV radiation, X-ray and gamma radiation.
- In Ionosphere, the temperature increases again as a result of the absorption of short-wave solar radiation by the atoms of oxygen and nitrogen.
Ionosphere consists of the following layers
- D layer (lowest)- It reflects low-frequency radio waves but absorbs medium and high-frequency waves.
- E Layer- It is also called “Kennelly-Heaviside” layer. It reflects medium and high-frequency radio waves. It is much better defined than the D-layer.
- F Layer- It is called the Appleton layer. This layer is especially important in long-distance radio communication. It reflects the medium and high-frequency radio waves.
- G-Layer- It is present much of the time, but it is not detectable, because the F layer reflects all waves reflected by this layer.
Exosphere
- The outermost layer of the earth’s atmosphere is known as the exosphere which lies between 400-1000km.
- The atmosphere in this region is so rarefied that it resembles a nebula.
- Hydrogen and helium gases predominate in this outermost region.
- At the outermost boundary of our atmosphere, the kinetic temperatures may reach to about 55700C.
- However, this temperature in its real sense is entirely different from the temperatures at the earth’s surface.
Modern views regarding the structure of the earth’s atmosphere
Based on composition, the earth’s atmosphere is divided into two broad categories-
Homosphere
- This is the lower part of the atmosphere which extends up to a height of about 88 km.
- It has uniformity in composition, that is, the proportions of the component gases of this sphere are uniform at different levels.
- It includes Troposphere, Stratosphere, Ozonosphere and Mesosphere.
Heterosphere
- The atmosphere above homosphere is not uniform in its composition, hence the name heterosphere.
- Different layers in this part differ from one another in their chemical and physical properties.
- The heterosphere is also referred to as the “Thermosphere” because the temperature goes on rising to the outermost boundary.
- In the upper atmosphere, the high temperature is due to the photochemical actions of the ultra-violet solar radiation.
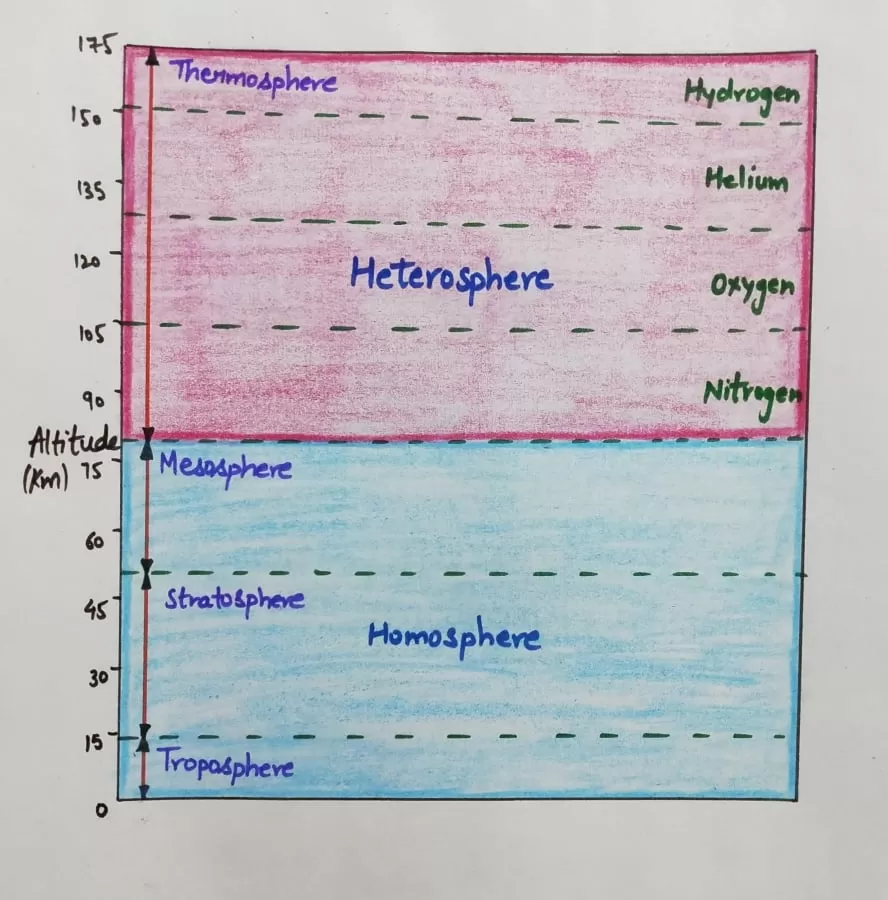
Gases arranged in the Heterosphere
- Nitrogen layer- This is the lowermost layer consisting of Molecular nitrogen extending from 88km-200km.
- Ozone layer- This layer comprises atomic oxygen extending between 200km-1120kms.
- Helium layer- This layer comprises Helium, extends between 1120km-3520km. At the top, there is the hydrogen layer extending up to the outermost boundary of our atmosphere.
- Hydrogen layer- The topmost layer in the heterosphere.
Read more
- How did the theory of plate tectonics evolve over time?

- 7 Criticisms of Continental Drift Theory by Alfred Wegener

- Malthus theory of population: critical analysis & relevance

- Major Types of Clouds formation and their Characteristics

- Get Detailed Bpsc syllabus 2022-23

- Complete bpsc geography optional syllabus 2022






Pingback: Bpsc geography optional syllabus 2022 updated | geography4u